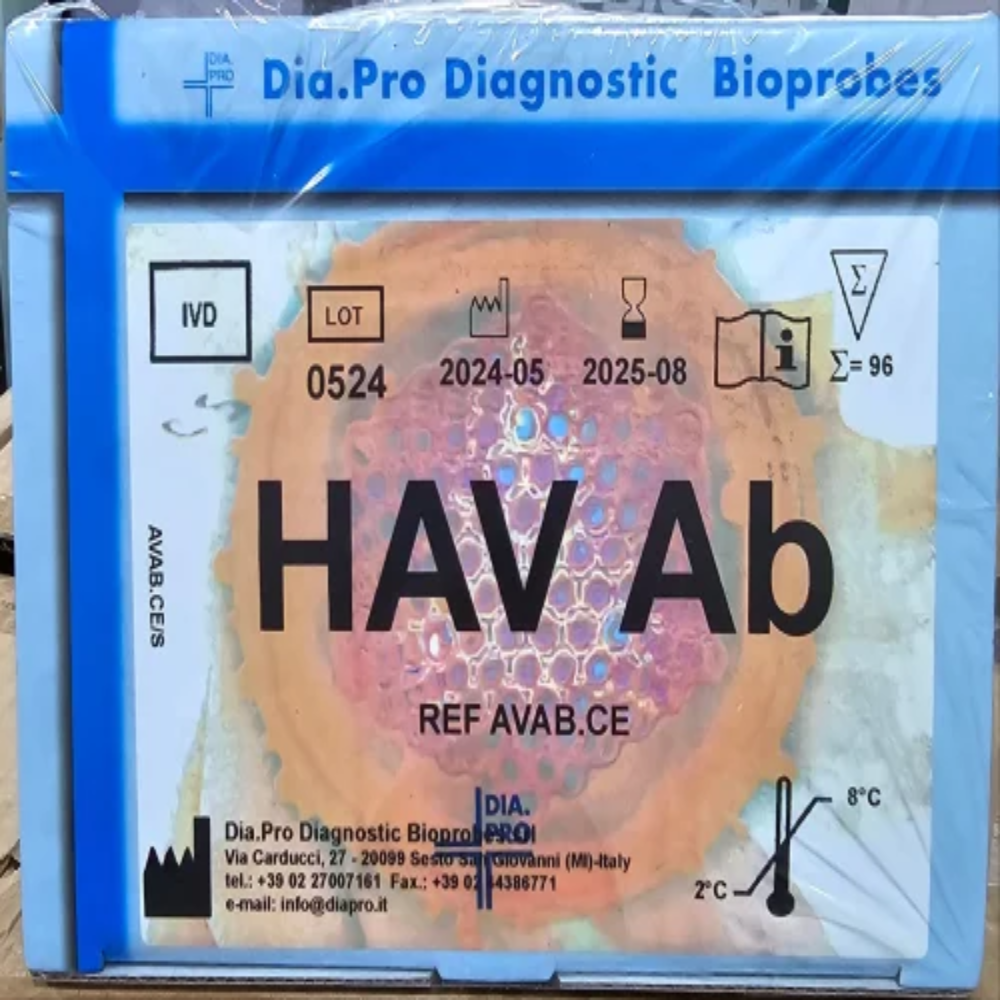
DIAPRO HAV AB ELISA KIT
Product Details:
DIAPRO HAV AB ELISA KIT Price And Quantity
- 6000 INR/Piece
- 10 Piece
Product Description
Dia.Pro HAV Ab ELISA KitThe DIAPRO HAV AB ELISA Kit is a competitive enzyme immunoassay (ELISA) designed to detect antibodies to Hepatitis A Virus (HAV) in human plasma and sera. The kit is used for the follow-up of patients infected by HAV.
Key Features:- Competitive ELISA format
- Detects antibodies to HAV in human plasma and sera
- Used for the follow-up of patients infected by HAV
- In vitro diagnostic use only
The assay is based on the principle of competition, where the antibodies in the sample compete with an anti-HAV conjugate for binding to a fixed amount of HAV antigen coated onto the microtiter plate wells. The amount of conjugate bound is inversely proportional to the amount of antibodies to HAV present in the sample.
Components:- Microtiter plate coated with HAV antigen
- Anti-HAV conjugate
- Sample diluent
- Wash buffer
- Substrate/chromogen mixture
- Stop solution
- Add sample diluent to the sample and mix well.
- Add 100 L of the sample to the corresponding well of the microtiter plate.
- Add 50 L of anti-HAV conjugate to each well.
- Incubate for 1 hour at room temperature.
- Wash the wells with wash buffer.
- Add 100 L of substrate/chromogen mixture to each well.
- Incubate for 30 minutes at room temperature.
- Add 50 L of stop solution to each well.
- Measure the optical density (OD) at 450 nm using an ELISA reader.
The OD value is inversely proportional to the amount of antibodies to HAV present in the sample. A positive result indicates the presence of antibodies to HAV, while a negative result indicates the absence of detectable antibodies.
Note:The DIAPRO HAV AB ELISA Kit is intended for in vitro diagnostic use only and should be used by trained laboratory personnel according to the manufacturer™s instructions.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+





 Send Inquiry
Send Inquiry Send SMS
Send SMS Call Me Free
Call Me Free
